Percutaneous intervention during pregnancy of severe coarctation of the aorta
Heba Nashat 1,2, Isma Rafiq 1,2
Department of Adult Congenital Heart disease, Royal Brompton Hospital, London, United Kingdom
National Heart and Lung Institute, Imperial College London, United Kingdom
Case Presentation
A 27 year woman was referred to our centre for management of severe coarcation of the aorta ( CoA) which was diagnosed incidentally when she presented with a subarachnoid haemorrhage (SAH) to her local hospital. She had a history of hypertension when she was 20 years old, without further investigations for secondary causes. She subsequently had 2 successful pregnancies, both complicated by hypertension and foetal growth restriction. Whilst awaiting percutaneous intervention, she became pregnant and declared this at 12 weeks gestation. The patient made an informed decision to continue with the pregnancy. At this time, extremity blood pressure in the right arm was 160/91mmHg and 89/51mmHg in the right leg. On examination there was a loud coarse ejection systolic murmur and lower limb pulses were difficult to palpate. She was commenced on methyldopa 125mg TDS and Aspirin 150mg and continued on amlodipine 10mg which she was previously taking.
Investigations
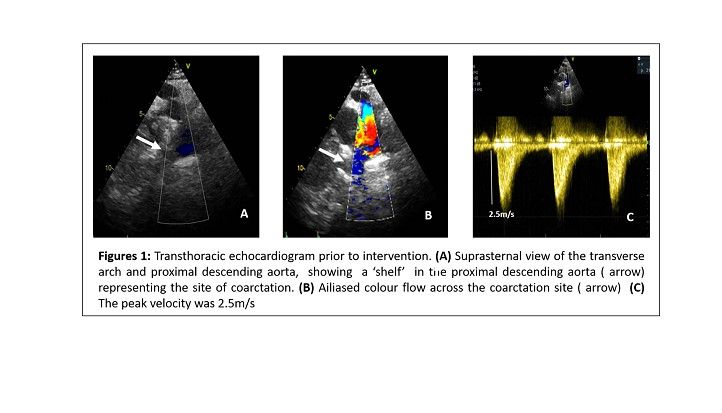
Full blood count, biochemistry assay and urinary protein-creatinine ratio were all unremarkable. Aortic knuckle with rib notching was identified on chest X-ray. An echocardiogram showed moderate left ventricular hypertrophy with significant diastolic dysfunction, tri-leaflet aortic valve with subaortic acceleration without significant obstruction and flow velocity in the descending aorta measuring 2.5m/s (Figure 1). A cardiac CT showed severe coarctation of the aorta with short segment, severe narrowing of the descending aorta distal to the origin of the left subclavian artery and ligamentum arteriosum and collateral pathways. The aorta measured 4mm in diameter at the its maximum stenotic point when compared to 18mm in the proximal segment. There was collateralisation to the distal segment through intercostal and vertebral arteries. At 20 weeks gestation a foetal echocardiogram showed CoA, parachute mitral valve and left ventricular/ right ventricular disproportion in keeping with a Shone syndrome.
Management
At 24 weeks gestation, now on amlodipine 10mg OD and methyldopa 500mg TDS, she remained significantly hypertensive. A multi-disciplinary team meeting considered that with a recent SAH, uncontrolled hypertension and a foetus with significant congenital heart defects, intra-partum relief of the CoA was necessary for optimum maternal and foetal outcome.
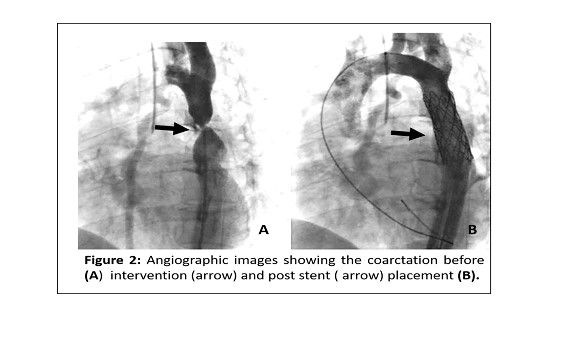
Cardiac catheterisation was performed with the patient under general anaesthesia with lead shielding of the uterus. Distal aortic measurements confirmed significant narrowing and were as follows; pre-coarctation 11mm, CoA site 4mm and post coarctation 14mm. A 4.5cm covered stent on a 14mm x 5cm delivery balloon was implanted. The pre-intervention pressure gradient was 32mmHg which reduced to 5mmHg after intervention (Figure 2). The total fluoroscopy time was 8 minutes and the patient received approximately 2mGy of radiation. Her immediate post procedure course was complicated by a hematoma at the puncture site which was managed conservatively. The pregnancy continued without complications with almost normalisation of her blood pressure resulting in a need to reduce the amlodipine to 5mg and discontinue the methyldopa. Antenatal screening of the foetus after the intervention, showed adequate growth of cardiac structures and normalisation of the LV/RV disproportion. She underwent an elective caesarean section at 38 weeks gestation due to fetal growth restriction. A 2,700mg male was delivered and transferred to a neonatal cardiac centre and had surgical intervention for his severe CoA. Her post-delivery recovery was unremarkable and at one year follow-up the patient remained stable.
Discussion
Coarctation of the aorta is defined as a congenital narrowing of the aorta, usually at the level of the isthmus in the majority of patients, but rarely can occur along the thoracic or abdominal aorta. It accounts for 5 to 8% of all congenital heart defects. Up to 20% of patients with CoA present as adults, sometimes in the form of treatment resistant hypertension, as demonstrated in this case. Associated lesions are not uncommon with CoA, most commonly bicuspid aortic valve in up to 85% , others include aortic aneurysm, sub or supra aortic stenosis or mitral valve stenosis.
Imaging modalities
Echocardiography is the commonest imaging modality used in both diagnosis and screening, by providing information regarding the site, extent of the CoA and associated congenital defects. Technically, both supra sternal and high parasternal short-axis views are required to visualise the arch clearly.[1] Inner edge to inner edge measured dimensions are taken in systole and should be indexed to body surface area. Doppler gradients on echocardiography are least reliable, particularly in the presence of extensive collaterals or post repair due to changes in aortic compliance, which may overestimate the gradient. However, a diastolic tail in the descending aorta and diastolic forward flow in the abdominal aorta usually indicate significant gradient.
In CoA, the preferred non-invasive imaging technique is CMR or CT, with 3D reconstruction. These imaging modalities evaluate the entire aorta and are best for detecting important complications such as aneurysms, pseudo aneurysms and restenosis. [2] CMR is a reliable and cost effective technique in both native and follow-up imaging. Moreover, CMR is increasingly seen to be the imaging method of choice as it yields both structural and functional assessment, unlike CT which provides only structural assessment.
The recommendations for intervention in CoA patients relies on the degree of arterial hypertension and invasive peak-to-peak gradient measured by cardiac catheterization. Intervention is recommended to all who have a peak-to-peak gradient ≥ 20mmHg who are hypertensive (class I recommendation). In a normotensive patient with a peak-to-peak gradient ≥20mmHg, a ≥50% narrowing relative to the aortic diameter at the diaphragm supports intervention. Hypertensive patients with a ≥50%narrowing relative to the aortic diameter but with a peak-to-peak gradient of <20mmHg should also be considered if technically feasible. [3] In adults with native or re-coarctation, stenting is the treatment of choice. Covered stents are chosen modalities as they are also very useful in covering for dissections which are a recognisable complication of the procedure. Balloon angioplasty is reserved to re-dilate previously stented aortas. [4]
Pregnancy and CoA
The modified WHO classification of maternal pregnancy risk, stratifies those with CoA at moderately increased risk (mWHO II-III) and those with severe CoA at the highest risk (mWHO IV). [5] Conversely, in a recent study on pregnancy outcomes in 303 women in the ROPAC registry has suggested that CoA may be better tolerated than previously appreciated. [6] Nevertheless, childbearing women should be counselled appropriately, and offer termination of pregnancy in those at significant risk of complications. Those who continue with the pregnancy should be managed in centres equipped with expertise in both pregnancy and cardiac diseases, by a multidisciplinary approach.
This case demonstrates the successful percutaneous repair of a significant CoA during pregnancy. In the gravid state, high oestrogen levels can impact on aortic remodelling, further aggravating coarctation-associated aortopathy and hypertension thus potentially increasing the risk of aortic rupture or dissection. [7] Unlike the non-pregnant state, the CoA gradient is less considered, but rather invasive intervention of CoA should be offered to pregnant women who have refractory hypertension or maternal or foetal compromise. [8] Covered stents are preferable over balloon angioplasty alone. Those with chronic hypertension are at increased risk of pre-eclampsia and should be offered low dose aspirin (100-150mg OD) from 12 weeks gestation. Initiation or optimisation of pharmacotherapy for hypertension is indicated when the blood pressure is ≥ 150/95mmHg, this threshold is lowered to >140/90mmHg in those with pre-existing hypertension or end organ damage. Methyldopa, labetalol and calcium antagonists are the treatments of choice in pregnancy.
Ionising radiation exposure during pregnancy is always heavily considered. Exposure in the first trimester pose the highest risk to organogenesis. Typically, doses of 100-200mGy are associated with radiation induced abnormalities such as growth restriction, intellectual disability and neurological malformations, whereas 20mGy may increase the risk of childhood malignancy. During catheterisation, the radiation exposure to the unshielded abdomen is 1.5mGy and <20% reaches the foetus. According to the 2018 ESC pregnancy guidelines, radiation should be kept to an absolute minimum, and not more than 50mGy if possible. [8]
Key Learning points
⦁ Cardiac MRI is the imaging technique of choice in the assessment of both native and post-repair CoA. Echocardiography is important in diagnosing or routine surveillance of associated congenital defects.
⦁ Severe CoA in pregnancy presents the highest maternal and foetal risks and all child bearing women with CoA should be appropriately counselled.
⦁ Intervention hinges on the degree of arterial hypertension, degree of narrowing and invasive peak-to-peak gradient.
⦁ Percutaneous intervention with a covered stent is the treatment of choice when indicated and if technically feasible.
Multiple choice questions
⦁ According to the modified WHO classification of maternal pregnancy risk, patients with severe coarctation of the aorta are stratified as;
⦁ WHO I
⦁ WHO II
⦁ WHO III
⦁ WHO IV
⦁ When assessing coarcation of the aorta by echocardiography, doppler gradients are reliable.
⦁ True
⦁ False
⦁ The imaging technique of choice when assessing coarcation of the aorta is
⦁ Computed tomography (CT)
⦁ Echocardiography
⦁ Cardiac magnetic resonance imaging (CMRI)
⦁ Chest X-ray
⦁ Intervention for coarctation of aorta should be offered to a patient if the invasive peak gradient is;
⦁ >5mmHg
⦁ >10mmHg
⦁ >15mmHg
⦁ >20 mmHg
⦁ In pregnant patients with coarctation of the aorta, the following statements are false.
⦁ Intervention should be offered if refractory hypertension
⦁ Intervention should be offered if signs of maternal compromise
⦁ Intervention should be offered if foetal compromise
⦁ Intervention should only be offered if the gradient is >20mmHg
⦁ In pregnancy the CoA gradient is less considered for intervention
References
[1] S. P. Goudar, S. S. Shah, and G. S. Shirali, ‘Echocardiography of coarctation of the aorta, aortic arch hypoplasia, and arch interruption: strategies for evaluation of the aortic arch’, Cardiol. Young, vol. 26, no. 8, pp. 1553–1562, Dec. 2016, doi: 10.1017/S1047951116001670.
[2] B. Shepherd et al., ‘MRI in adult patients with aortic coarctation: diagnosis and follow-up’, Clin. Radiol., vol. 70, no. 4, pp. 433–445, Apr. 2015, doi: 10.1016/j.crad.2014.12.005.
[3] H. Baumgartner et al., ‘2020 ESC Guidelines for the management of adult congenital heart diseaseThe Task Force for the management of adult congenital heart disease of the European Society of Cardiology (ESC)’, Eur. Heart J., doi: 10.1093/eurheartj/ehaa554.
[4] Taggart Nathaniel W. et al., ‘Immediate Outcomes of Covered Stent Placement for Treatment or Prevention of Aortic Wall Injury Associated With Coarctation of the Aorta (COAST II)’, JACC Cardiovasc. Interv., vol. 9, no. 5, pp. 484–493, Mar. 2016, doi: 10.1016/j.jcin.2015.11.038.
[5] I. M. van Hagen and J. W. Roos-Hesselink, ‘Pregnancy in congenital heart disease: risk prediction and counselling’, Heart, vol. 106, no. 23, pp. 1853–1861, Dec. 2020, doi: 10.1136/heartjnl-2019-314702.
[6] K. P. Ramlakhan et al., ‘Pregnancy outcomes in women with aortic coarctation’, Heart, Oct. 2020, doi: 10.1136/heartjnl-2020-317513.
[7] ‘Management of Pregnancy in Patients With Complex Congenital Heart Disease: A Scientific Statement for Healthcare Professionals From the American Heart Association | Circulation’. https://www.ahajournals.org/doi/10.1161/CIR.0000000000000458 (accessed Jan. 26, 2021).
[8] V. Regitz-Zagrosek et al., ‘2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy: The Task Force for the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC)’, Eur. Heart J., vol. 39, no. 34, pp. 3165–3241, Sep. 2018, doi: 10.1093/eurheartj/ehy340.
